
THE PEPTAMEN® FAMILY IS SUPPORTED BY OVER 50 PUBLICATIONS. WE HAVE SELECTED FOUR KEY SUPPORTING EVIDENTIAL PIECES, SPECIFICALLY FOR PAEDIATRICS TO SHARE WITH YOU IN DETAIL, AS WELL AS HIGHLIGHTS FROM OUR RANGE OF CLINICAL EVIDENCE.
Study 1: Formula switch leads to enteral feeding tolerance improvements in children with developmental delays.
Study 2: Addition of fibre to an elemental diet is well tolerated.
Study 3: Incidence of gastroesophageal reflux with whey and casein-based formulas in infants and in children with severe neurological impairment.
Case Study: The 4-year journey of feeding intolerance of an enterally-fed child from 9 months of age.
Study 1
Formula Switch Leads to Enteral Feeding Tolerance Improvements in Children with Developmental Delays.
Minor G, Ochoa JB, Storm H, Periman S. (2016). Global Paediatric Health. 3, 1-6.
Background:
Children with developmental delay commonly experience poor tolerance to enteral feeding, often due to gastrointestinal dysmotility. Intolerance to enteral feeding may prevent nutritional goals from being met, and may impact negatively on a child’s growth and development.
Objectives:
The study aimed to evaluate changes in tolerance parameters when enterally fed children with developmental delay were switched from an intact protein formula to a 100% whey peptide formula.
Design:
Retrospective chart review.
Patients:
13 children with developmental delay, aged 8.4 +/- 4.6 years.
Method:
Children were switched to one of the following 100% whey peptide formulas from an intact protein formula.
- Peptamen® Junior (n=6).
- Peptamen® Junior 1.5 (n=6).
- Peptamen® Junior Prebio (n=1).
Outcomes measured:
- Vomiting.
- Gagging and retching.
- High gastric residual volumes.
- Constipation.
- Diarrhoea.
- Poor weight gain.
- Use of medications to manage feeding intolerance (e.g. prokinetics).
Results:
- 92% of children (12/13 patients) showed improvement in feeding tolerance attributed to switching to a 100% whey peptide formula.
- Of these, 75% (9/12 patients) reported that improvements occurred within one week of the formula change.
- Improvements in vomiting (86%), gagging and retching (75%), high gastric residual volumes (63%), constipation (43%) and diarrhoea (100%) were noted in those who had specific intolerance symptoms.
- Of those patients who were receiving medications to manage feeding intolerance, 81.8% either reduced their dosage or stopped medications completely following a switch to a 100% whey peptide formula.
- 71% of subjects were able to tolerate an increase in feed volume.
- All subjects who had experienced poor weight gain showed an increase in weight following the formula change.
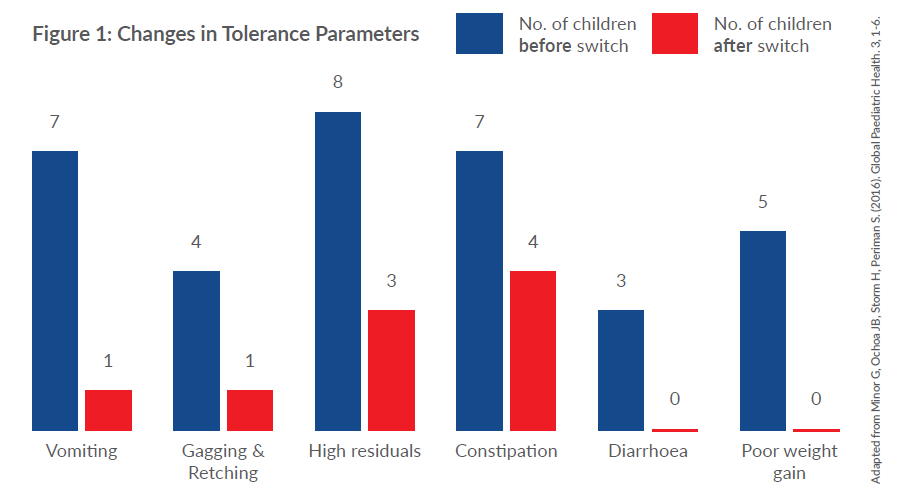
Conclusion:
In children with developmental delay, switching to a 100% whey peptide formula from an intact protein formula improved symptoms of feeding intolerance. The change in formula was associated with improved growth and a reduction in the use of medications to manage feeding intolerance.
Study 2
Tolerance of an Enteral Formula with Insoluble and Prebiotic Fibre in Children with Compromised Gastrointestinal Function.
Khoshoo V, Sun S , Storm H. (2010). J Am Diet Assoc. 110: 1728-1733.
Background:
In patients with gastrointestinal dysfunction, it has not been common practice to use a fibre-containing enteral formula. This is despite the important roles that soluble and insoluble fibre can play in the support of gut integrity and improvement of stool consistency.
Objectives:
To assess the tolerance, stool frequency and stool consistency of children receiving a fibre-free peptide feed (Peptamen Junior®) compared to Peptamen Junior® with the addition of 3.5g FOS and 3.8g insoluble fibre per litre.
Design:
6 week randomised, double blind–cross-over trial.
Patients:
14 children aged 1-14 years with diagnoses of gastrointestinal dysmotility (n= 9), Crohn’s disease (n= 3), or mild short bowel syndrome (n= 2).
Method:
The following diet sequence was applied to all patients:
- 5 day run-in period with an unflavoured fibre-free formula, similar to the control formula.
- Switch to either the fibre-containing peptide feed or the fibre-free control formula (Peptamen® Junior) for 14 days.
- 5 day washout period.
- Switch to the second diet for another 2 weeks of feeding.
Outcomes measured:
Primary outcomes:
- Daily stool frequency (number of stools passed per 24 hours).
- Stool consistency (nuts/lumps/sausage/snakes/mush/fluffy/watery).
Secondary outcomes:
- Vomiting.
- Abdominal pain.
- Abdominal distension.
- Flatulence.
- Feed intakes.
Results:
- All children met their goal feeding volumes on both formulas.
- Stool frequency did not differ by formula.
- Subjects reported more “fluffy” stools (p< 0.001) and less watery stools (p< 0.01) with the fibre-containing formula compared to the control formula.
- There were no significant differences in vomiting, abdominal pain, or feeding intakes between formulas.
Conclusion:
The addition of FOS and insoluble fibre to Peptamen Junior® was well tolerated and resulted in significantly fewer watery stools than Peptamen Junior® without fibre.
Study 3
Incidence of Gastroesophageal Reflux with Whey and Casein- Based Formulas in Infants and in Children with Severe Neurological Impairment.
Khoshoo V, Zembo M, King A, Dhar M, Reifen R, Pencharz P. (1996). J Pediatr Gastroenterol Nutr. 22(1):48-55.
Background:
Gastroesophageal reflux (GOR) is a common occurrence in infants and children with cerebral palsy, and whey-based formulas have been shown to promote faster gastric emptying, leading to fewer vomiting episodes. Prior to this study, it was not known whether faster gastric emptying with whey-based formulas also resulted in fewer episodes of GOR in these children.
Objective:
To determine whether whey-based formulas are associated with fewer episodes of GOR compared to a casein-based formula in neurologically impaired children.
Design:
Randomised, cross-over clinical trial.
Patients:
10 children (4.5-14.5 years old):
- With severe cerebral palsy, profound developmental delay and neuromuscular impairment.
- With clinical evidence of GOR (defined as more than two episodes of effortless regurgitation per 24 hours).
- Exclusively fed through a gastrostomy tube placed more than 3 months ago.
Method:
- Casein predominant formula: Osmolite® (Abbott).
- Whey predominant formula: Peptamen®.
- Each child received each formula for 48 hours and then crossed over to the other formula. The first 24h period on each formula was regarded as the washout period. Measurements were undertaken in the second 24h period for each formula.
Outcomes measured:
- Number of episodes of GOR per 24h.
- Duration of time that the pH in the distal oesophagus was below 4.
Results:
- The number of reflux episodes in individual patients was significantly reduced (p<0.05) while on the whey-based formula compared to the casein-based formula.
- When the results of group means were compared, a significant reduction in frequency of GOR was shown with the whey-based formula (51 +/- 15.7 episodes) compared to the casein-based formula (73.1 +/- 22.8 episodes) (p<0.05).
- The oesophageal pH was below 4 for a significantly shorter duration (p< 0.02) during the whey-based feeding as compared to casein-based feeding in individual patients.
- A significant reduction in the mean duration of GOR was also observed with the whey-based formula compared to the casein-based formula (p<0.05).
Conclusion:
A whey-based formula resulted in a reduction in episodes and duration of gastroesophageal reflux.
“Whey-based formulas should be considered as an additional tool in conjunction with other anti-reflux measures to treat gastroesophageal reflux more effectively in children with severe neurological impairment.”
The 4-Year Journey of Feeding Intolerance of an Enterally-Fed Child from 9 Months of Age.
Emma Liesl Silbernagl, data on file: PEP047 Dec 16
Background:
‘Child M’: born premature at 33 weeks and 4 days with a complex secondary diagnosis that included GORD, failure to thrive, intrauterine growth restriction, vitamin D resistant rickets, abnormal vocal cords and chronic lung disease.
Aims/objective:
- Ensure catch-up growth and subsequent healthy weight-gain achieved (tracking on 25th centile, in line with length).
- Feeding tolerance and moving towards safe oral intake.
- To meet nutritional requirements 120-130 kcal/kg/day and 120ml/kg/day to achieve catch-up growth and maintain hydration status.
Nutritional problems:
- Poor tolerance to oral feeds.
- Frequent watery stools (non-infected).
Nutritional interventions:
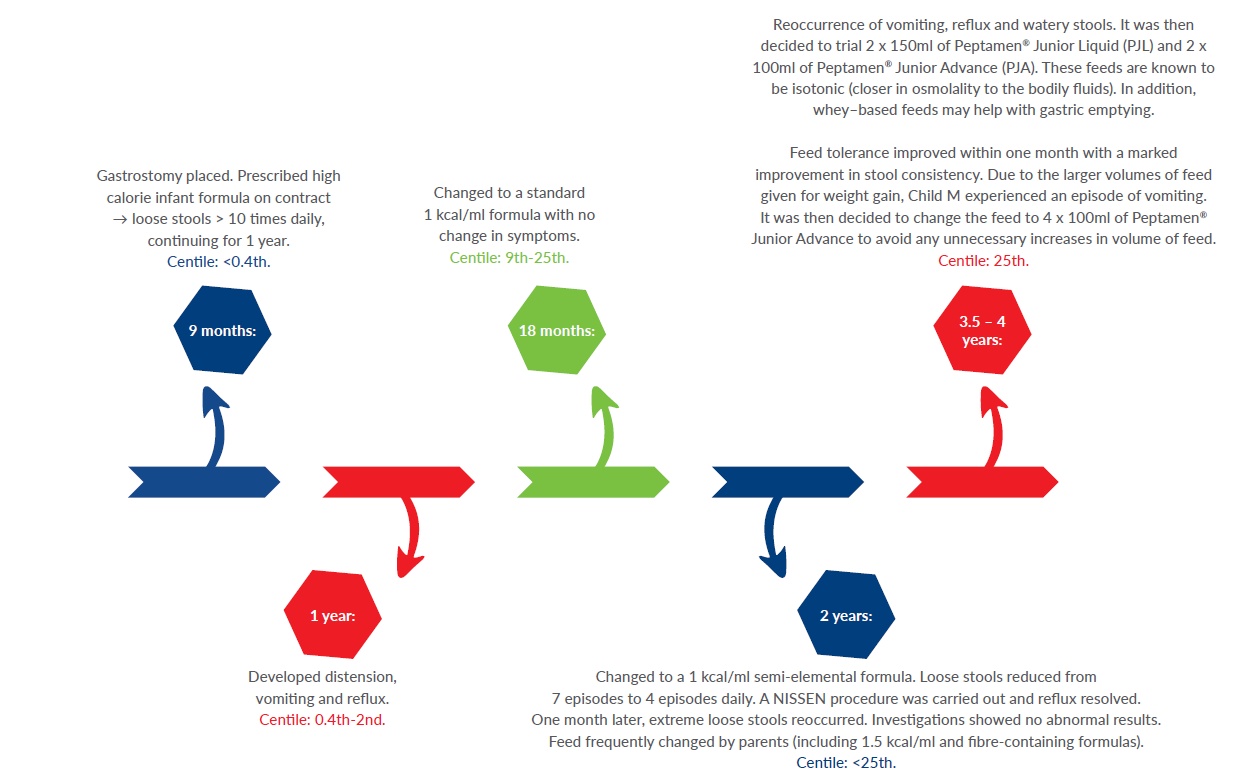
Conclusion:
Child M is now 5 years of age, tolerating 4 feeds a day and her weight is now between the 25th–50th centile. Her mum reported that she saw the first normal ‘poo’ after many years of struggling. Her quality of life has improved and she is able to partake in more school activities.
For a full copy of this case study please contact your local representative.
Clinical evidence summary
|
Authors and Journal |
Patient conditions |
Formulas studied |
Study objectives |
Study objectives |
|
Fried MD et al. Journal of Pediatrics. 1992; 120 (4):569–72. |
Paediatric patients with spastic quadriplegia and documented delayed gastric emptying. |
Casein-predominant vs. Whey-predominant (including Peptamen®).
|
To determine gastric emptying times and incidence of regurgitation in children with documented delayed gastric emptying. |
Patients on whey-based formulas had a significant reduction (p<0.05) in vomiting (mean 2±2 episodes) compared with those on the casein-based (mean 12±11 episodes). Whey-based formulas like Peptamen® reduce the frequency of vomiting by improving the rate of gastric emptying (p<0.001). |
|
Khoshoo V et al. European Journal of Clinical Nutrition. 2002; 56:656-658. |
Paediatric gastrostomy-fed patients with volume intolerance.
|
Peptamen® 1.5 vs. Peptamen®. |
To study the emptying rates of equal volumes of two similar whey-based formulas of different energy densityand clinical implicationsin children with volume intolerance. |
Gastric emptying of the two formulas was similar (p>0.05). There was significantly more weight gain with Peptamen® 1.5 (mean 1.17 +/- 0.5kg) after one month of feeding (p<0.05). Peptamen® and Peptamen® 1.5 were equally well tolerated. However, energy intake may be optimised with the more calorically-dense product, Peptamen® 1.5, in this patient population. |
|
Dylewski ML et al. Nutrition Poster 72; A.S.P.E.N. Clinical Nutrition Week. 2006. |
Paediatric patients with burns exceeding 20% TBSA. |
Peptamen® vs. standard casein-based formula. |
To compare the effects of a whey-based peptide formula (Peptamen®) vs. an intact casein-based formula in paediatric burn patients. |
Peptamen® is better tolerated than the casein-based feeding in paediatric burn patients. Peptamen® promoted more rapid progression to goal feeding and a decrease in incidence of diarrhoea (p=0.03). |
|
Khoshoo V et al. (1996) Pediatr Gastroenterol Nutr. 22(1):48-55. |
Children with severe cerebral palsy with GOR and frequent episodes of vomiting. |
Peptamen® vs casein predominant. |
To determine the incidence of gastroesophageal reflux with whey and casein-based formulas in infants and in children with severe neurological impairment. |
There was a significant reduction (p< 0.05) in the number of episodes of reflux on whey-based formula compared to the casein-based formula. A significant reduction in frequency of GOR with whey-based feeds (51 ± 5.7 episodes) and casein (73.1 ± 22.8 episodes) (p< 0.05). There was also a significant reduction (p< 0.05) in the mean duration of GOR in the whey group as compared to the casein group. |
|
Hampsey J et al. (1997). Journal of the Federation of American Societies for Experimental Biology. 11(3):1085A. |
Symptomatic HIV infected children. |
Provided 227ml of Peptamen® Junior daily for 6 months. |
Early enteral nutrition intervention with HIV infected children. |
Mean weight Z score increased from 0.55 (+/-0.51) to 0.89 (+/- 0.49), (p= 0.059). Weight gain velocity was significantly greater during the study vs before the study (p= 0.047), however no improvement was observed in linear growth velocity. Supplementation resulted in significantly increases in triceps skinfold measurement (9 ± 1 mm vs. 12 ± 1mm; p= 0.021), but there was no change in arm muscle area. Children who consumed 227ml for >65% of the time throughout the study (n= 6) increased or maintained haematocrit of 35% and haemoglobin of 12g/dl, when compared to those that were less compliant (n= 4) (p= 0.005). |
|
Erskine JM, Lingard CD, Sontag MK, Accurso FJ. (1998). J Pediatr. 132(2):265- 9. |
Children with cystic fibrosis and pancreatic insufficiency and had enzyme replacement therapy for at least 3 months. |
Peptamen® without enzyme replacement vs. a standard enteral formula plus usual dose of enzyme therapy. |
A comparison of a semi-elemental and non-elemental formula in patients with cystic fibrosis. |
Weight gain was not different between groups (p= 0.92). There was no difference in the coefficient of fat absorption between groups (p = 0.58). Similarly, there was no difference in the coefficient of nitrogen absorption for the Peptamen® and Isocal® studies (p= 0.48). |
|
Brackett K, Alexander J, Fowler RD (2000). Nutrition Intervention in Gastrointestinal Motility Presented at the 24th ASPEN Clinical Congress, Nashville, USA. |
A premature born baby who was also diagnosed with gastro-oesophageal reflux (GOR) and delayed gastric emptying (DGE). |
Transition from a casein based formula to Peptamen® Junior. |
A case study on the nutritional intervention of a child who was born premature. |
Immediate cessation of vomiting was seen with a reduction in episodes from 14 per day to none. There was an improvement in oral feeding skills and tolerance to larger volumes of oral feeding. Accelerated weight gain and linear growth was seen. There were also improvements in stool pattern and enhanced participation in activities and therapies. |
|
Flack S, Lawson M, Milla P. (2003). J Hum Nutr Diet 16:365-70. |
Children requiring supplementary feeding. |
Peptamen® Junior. |
To evaluate the use and tolerance of Peptamen® Junior, a hydrolysed feed in a paediatric gastroenterology clinic. |
The mean change in weight after 28 days was +0.01 SDS. Seven out of 8 children (88%) with diarrhoea improved over the 28 days. All children with vomiting improved over the 28 days. Five out of the 6 children (83%) with abdominal pain improved over the 28 days. Nine out of 15 children (or parents) considered that their symptoms improved and they chose to continue with the trial feed. At follow up (2-5 months later) 4/9 children remaining on the trial feed showed positive changes in weight since starting the feed. |
|
Khoshoo V, Sun S, Storm H. (2010). J Pediatr Gastroenterol Nutr, Vol 43:E14-E76, Presented at NASPGHAN 2006. |
Children with diagnoses of gastrointestinal dysmotility, Crohn’s disease, or mild short bowel syndrome. |
Peptamen® Junior with fibre vs. Peptamen® Junior with no fibre. |
To assess stool frequency and consistency in children enterally fed an elemental diet containing fibre. |
All children maintained goal feeding volumes on both formulas. Stool frequency did not differ by formula. Subjects reported more “fluffy” stools (p< 0.001) and less watery stools (p< 0.01) when consuming Peptamen® Junior with fibre than when consuming Peptamen® Junior. Abdominal circumference and flatulence were also significantly lower with Peptamen® Junior with fibre than with Peptamen® Junior. No significant differences were observed in vomiting, abdominal pain, or feeding intakes between formulas. |
Clinical evidence summary
|
Authors |
Patient conditions |
Formulas studied |
Study objectives |
Study objectives |
|
Biancia Parau. |
A child with traumatic brain injury (TBI). |
PaediaSure (standard feed) vs. Peptamen® Junior powder. |
A case study on feed intolerance in a paediatric traumatic brain injury patient. |
This child experienced severe marasmus, weight loss and watery diarrhoea. The child was started on parenteral nutrition and weaned onto PaediaSure®. However once started on PaediaSure®, the child developed watery diarrhoea and was changed to Peptamen® Junior Powder. Nutritional requirements of the child for macro and micronutrients for age were met. • Appropriate, gradual weight gain was achieved. • Tolerance to feed was better, as volume was not increased but Peptamen® feed was made more calorie dense. • Bowel movements were normal. |
|
Sam Armstrong. |
A child with Acute Lymphoblastic Leukaemia (ALL). |
Nutrini® Energy Multifibre vs. Peptamen® Junior powder and Peptamen® Junior Advance. |
A case study on the use of a high energy peptide feed to aid feed intolerance and promote growth in a paediatric oncology patient. |
This 4 year old child, newly diagnosed with acute lymphoblastic leukaemia (ALL), experienced significant intolerance to feeds as a result of his intense chemotherapy treatment regimen. The child experienced abdominal pain, loose stools (diarrhoea), reduced appetite, nausea and vomiting. The current feed Nutrini® Energy Multifibre was not being tolerated and was unable to increase volume of feed to achieve calorie needs. The feed was changed over to Peptamen® Junior powder and the child’s nausea improved and did not experience any further vomiting. |
|
Elaine Mealey. |
A child with severe neurodisabilities and Hirschsprung’s disease. |
Nutrini® Peptisorb and Calogen (Nutricia®) vs. Peptamen® Junior Advance. |
A case study on the use of a peptide feed to resolve feeding intolerances in a complex paediatric case. |
A child with cerebral palsy and Hirschsprung’s disease experienced persistent loose stools (opening 5-6 times/day) which had been ongoing for the last 3 years. The child was started on Nutrini® Peptisorb and Calogen boluses. The current regimen was unable to meet full nutritional requirements and the child started to experience constipation. The feed was then changed to Peptamen® Junior Advance (PJA). From day one PJA was tolerated extremely well and his bowels were opening (loose) 1-2 times/day. |
Evidence in adults
|
Authors of journal |
Category |
Patient conditions |
Formulas studied |
Study objectives |
Results |
|
Parekh N. American College of Gastroenterology Annual Meeting Abstracts. 2006: S313–14, Abstract Number 776. |
Gastroenterology. |
Adult patients with intestinal failure undergoing intestinal rehabilitation. |
Peptamen® with Prebio 1 (fibre blend: Inulin, FOS). |
To describe the outcome from switching from a polymeric or semielemental formula to Peptamen® with Prebio. |
Three months of oral or enteral intake of Peptamen® with Prebio 1 (fibre blend: Inulin, FOS) may induce weight gain in patients with intestinal failure undergoing intestinal rehabilitation. |
|
Salomon SB et al. Journal of the American Dieteti Association. 1998;98:460–2. |
HIV. |
Adult HIV. |
Peptamen® vs. regular diet. |
To determine if a hydrolysed whey-based, high MCT diet would improve gastrointestinal tolerance and fat absorption in HIV-infected subjects. |
Patients with HIV tolerated Peptamen® well. Significant decrease in number of stool (p<0.01) was seen during the Peptamen® phase of the study, in addition to a significant decrease in faecal fat content of stool (p<0.019). |
|
Wakefield S et al. 34th ESPEN Congress, Barcelona, Spain. Sept 8-11, 2012;7(1):1-300. |
Chyle leak. |
Upper GI cancer surgery. |
Peptamen® vs. very low fat, oral diet enriched with MCT. |
To evaluate incidence of chyle leaks after change in surgical technique; length of stay in patients with chyle leaks; nutrition effect on recovery time. |
Patients with chyle leaks (73%) had significantly longer length of hospital stay (24 vs. 16 days; p=0.003). The majority of patients’ chyle leaks resolved with specialised oral (27%) or enteral nutrition therapy (72%). |
Peptamen® Junior Advance
- Increased energy and protein
- 1.5 kcal/ml
- 4.5g protein per 100ml
- 100% whey protein
- 60% of fat as MCT
- Osmolarity: 380mOsm/l
- FOS/Inulin fibre blend
- Omega 3 fatty acids (0.2g per 100ml)
- Available in 500ml SmartFlexTM collapsible semi-rigid bottles for tube feeding.
Peptamen® Junior
- Standard energy and protein
- 1 kcal/ml
- 3.0g protein per 100ml
- 100% whey protein
- 60% of fat as MCT
- Osmolarity: 319mOsm/l
- Omega 3 fatty acids (0.1g per 100ml)
Available in 500ml SmartFlexTM collapsible semi-rigid bottles for tube feeding.
Peptamen® Junior Powder
- Flexible caloric density
- 1-1.5 kcal/ml
- 3.0g protein per 100ml*
- 100% whey protein
- 53% of fat as MCT
- Osmolarity: 322mOsm/l*
- Halal certified
Nestlé Health Science produces a range of foods for special medical purposes for use under medical supervision used with patients requiring either an oral nutritional supplement or a sole source of nutrition. ® Reg. Trademark of Société des Produits Nestlé S.A. For healthcare professional use only.
THE PEPTAMEN® FAMILY IS SUPPORTED BY OVER 50 PUBLICATIONS. WE HAVE SELECTED FOUR KEY SUPPORTING EVIDENTIAL PIECES, SPECIFICALLY FOR PAEDIATRICS TO SHARE WITH YOU IN DETAIL, AS WELL AS HIGHLIGHTS FROM OUR RANGE OF CLINICAL EVIDENCE.













