
Synopsis:
This case study discusses the management of a patient with Motor Neurone Disease, who developed reflux and regurgitation on commencing gastrostomy bolus feeding. Despite the use of various enteral and oral feeds, his symptoms persisted, and he lost approximately 8% of his body weight in 9 months.
After commencing Peptamen® AF, a 100% whey peptide formula with 50% of fat from MCT, his symptoms resolved and his weight stabilised. He continues to tolerate enteral feeding well after 8 months on his new feed and reports his quality of life has improved
Introduction
Motor Neurone Disease (MND) is a degenerative neurological condition, characterised by progressive muscle weakness and wasting. Both the upper and lower motor neurones are affected, and the rate of disease progression is variable.
The degeneration of motor neurones leads to impaired mobility, speech, swallowing and breathing.
Patient’s background/medical history
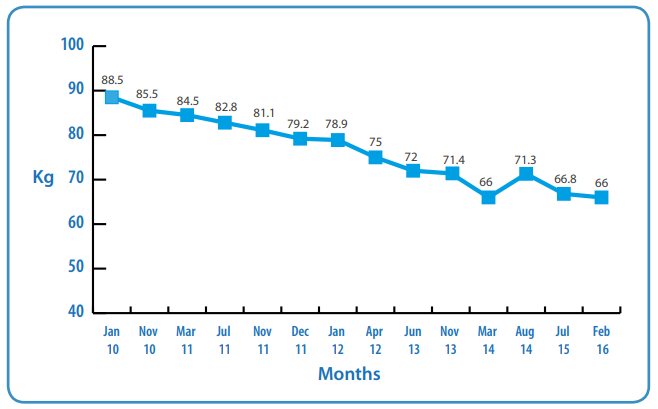
A 51 year-old male was diagnosed with Motor Neurone Disease (MND) slow progressing in September 2009. He had a history of Irritable Bowel Syndrome (IBS) and was referred to a Dietitian in January 2010. At this time his respiratory and physical function were stable and he lived with his wife and his 10 year-old daughter. There was no weight loss and he had deliberately gained weight (his usual weight was 83kg and his weight in January 2010 was 88.5kg). There were no reported difficulties with eating and drinking.
Nutritional Aims
• To minimise weight loss and meet nutritional requirements.
Medical and Dietetic Interventions
Nov 2010
Weight: 85.5kg. Visible loss of muscle mass was observed, however the patient was not concerned by this. A food-first approach was discussed.
Mar 2011
Weight 84.5kg. There had been a 4.5% weight loss since diagnosis. He was choking more and needed a texture modified diet, but his respiratory function was stable. Physical function and speech were impaired.
In view of the above, the pros and cons of a gastrostomy tube were discussed. The patient reported:
“Will have one if I need one. I am eating ok.”
July 2011
His weight was now 82.8kg, and it was noted that his dexterity had deteriorated. He was then placed on a soft blended diet, with fortified milk. His GP prescribed Fortisip® (Nutricia) 200ml twice daily.
Nov 2011
There was further weight loss, and he was not tolerating Fortisip or fortified milk. He was no longer able to feed himself and was dependent on his wife and carers at meal-times. Therefore gastrostomy tube insertion was discussed again.
Dec 2011
Weight was 79.2kg. He reported thinking about having a gastrostomy.
Jan 2012
Weight was 78.9kg. He again reported thinking about having a gastrostomy.
Apr 2012
His weight had now dropped to 75kg and he agreed to have a gastrostomy tube fitted. He was having difficulty swallowing liquids and medications, his meal-times were prolonged, and his increased choking episodes had made carers fearful of feeding him orally.
May 2012
A Corpak PEG 16Fr was fitted. The tube was only used for hydration as he was reluctant to use it for enteral feeding.
Jun 2013
Weight: 72kg. The PEG continued to be used to meet his fluid requirements only, as he remained reluctant to be enterally fed. He was no longer weight bearing and could not be weighed at home. He declined MND clinic visits as he found them too tiresome, but agreed to be weighed when he attended the hospice for symptom control.
A personal reflection on patients’ emotional barriers to gastrostomy feeding
During this time MND patients can go through various emotions and thoughts which should be considered when planning dietetic intervention.

July 2013
An unsuccessful trial of Fortisip® and Fresubin® Energy (with and without fibre) led the patient to agree to be bolus fed with Fresubin® 2 kcal (Fresenius Kabi a whole protein 2 kcal/ml formula). However, this was also not tolerated; the patient experienced reflux and regurgitation which made him feel sick. A speech and language assessment highlighted that he had an unsafe swallow and he was informed of the risks. The patient decided to continue to eat and drink at his own risk: he was on a texture D mashed diet and normal fluids through a straw.
Nov 2013
Weight 71.4kg. Due to ongoing gastrointestinal intolerance, the patient agreed to trial a peptide feed. He was prescibed 500ml of Survimed® OPD HN (Fresenius Kabi), a 1.33 kcal/ml peptide based feed with approximately 50% of fat as MCT via the feeding pump. The rate and time was dictated by the patient. He also included 1 sachet of powdered carbohydrate energy supplement (Maxijul, Nutricia) which was added to the water flushes. He continued to eat and drink small amounts at his own risk and lansoprazole was commenced.
Mar 2014
Weight 66kg. The patient agreed to increase feed to 750ml of Survimed® OPD HN and continued with 1 sachet of Maxijul added to water flushes. He was managing approximately 300-500 kcal/ day orally.
Nov 2014
By this time, the patient could not tolerate 750ml of Survimed® OPD HN and experienced reflux and regurgitation. Due to this, he found the taste unpleasant and it made him feel sick. The feed was reduced to 500ml administered with Maxijul and 3 x 30ml ProSource liquid protein supplement (Nutrinovo). Additionally, a multivitamin supplement and domperidone were prescribed. The patient continued to eat small amounts, drinking fluids. He acknowledged that he was not meeting his nutritional needs.
Aug 2015
The patient was not able to tolerate ProSource anymore as this made him gag, therefore it was discontinued. The other option was to try another 250ml of feed in the evening or 60g of skimmed milk powder added to 1000ml of cooled boiled water with Maxijul. He chose to have 60g of milk powder, but did not tolerate this.
Given the complexity of his ongoing intolerance issues, another range of peptide feeds was assessed. He agreed to try Peptamen® AF, a 1.5 kcal/ml formula with 100% whey peptides, omega-3 fats and 50% of the fat as MCT (Nestlé Health Science). He was able to tolerate 650ml of this feed. The patient was nil by mouth at this stage so it was important to try and meet his full nutritional requirements via the feeding tube.
Mar 2016
Between August 2015 and March 2016 the patient continued to tolerate 650ml of Peptamen® AF plus 1 sachet of Maxijul. In total, this regimen provided approximately 1500 kcal and 61g protein. This increased his nutritional intake by approximately 300 kcal and 28g protein per day.
The patient recognised he was not meeting his nutritional needs, however he did not wish to increase nutrition support at this time as his symptoms of reflux or regurgitation had resolved. Therefore the current aim was to maintain comfort and minimise weight loss. His most recent weight in February 2016 was 66 kg which was not concerning him. Although his general condition was deteriorating, he felt his quality of life had improved as his sickness and regurgitation subsided.
Weight Graph
Rationale for changing feed to Peptamen® AF
- Tried a range of feeds, rates and volumes which were tolerated to a variable degree.
- Despite the administration of domperidone and lansoprazole, reflux/gagging and nausea persisted.
- The range of available feeds on contract had all been tried, without success.
- Symptoms were impacting on the patient’s quality of life. The patient experienced reflux and regurgitation throughout the day with previous feeds.
- Around this time, the Nestlé Health Science Representative joined our team meeting detailing the range of Peptamen® feeds and their role in the management of gastrointestinal intolerance.
- A trial of Peptamen® AF was agreed, which was tolerated well
- Studies on whey-based feeds have shown a reduction in the incidence of reflux and gastro-oesophageal reflux disease in children with severe neurological impairment, therefore the same benefits may be applicable to adults.2
- Tolerance to volume was an initial issue, which seemed to resolve on Peptamen® AF.
- Peptamen® AF has a higher energy density compared to Survimed® OPD HN (1.5 kcal/ml vs 1.33 kcal/ml). A study has shown that gastric residuals were similar in whey-based formulas with different energy densities without affecting tolerance.3
Discussion
Delayed gastric emptying has been observed in patients with MND, as illustrated in this case study.4
For 6 months now, Peptamen® AF has continued to be tolerated by the patient with no further episodes of reflux, gagging or sickness. Due to the higher calorie and protein content in Peptamen® AF, his nutritional intake has significantly improved. The whey peptides may have helped with reflux due to faster gastric emptying.2
Conclusion
The nutritional management of MND patients can be challenging, especially if they have cognitive impairment. They do not have control of what is happening to them; however they do have control over the amount nutrition and hydration administered via their feeding tube. The multi-disciplinary team work closely together with patients to maximise comfort and to relieve symptoms, as well as physiological and psychological stress.
In Leeds we have a city wide contract with Fresenius Kabi and are obliged to use their range of feeds first. Upon reflection, I will now consider using Peptamen® AF sooner if patients are having tolerance issues.
References
1. Heffernan C, Holmes T, Feder G, Kupfer R, Leigh PN, McGowan S, Rio A and Sidhu P. (2004). Nutritional management in MND/ALS patients: an evidence based review. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 5, 72-83.
2. Khoshoo V, Zembo M, King, A, Dhar M, Reifen R, Pencharz P. (1996). Incidence of gastroesophageal reflux with whey- and casein- based formulas in infants and children with severe neurological impairment. Journal of Pediatric Gastroenterology & Nutrition 22 (1), 48-55.
3. Khoshoo V and Brown S. (2002). Gastric emptying of two whey-based formulas of different energy density and its clinical implications in children with volume intolerance. European Journal Clinical Nutrition 56, 656-658.
4. Toepfer, M, Folwaczny C, Lochmuller H, Schroeder M, Riepl RL, Pongratz D, and Muller-Felber W. (1999). Noninvasive (13)C-octanoic acid breath test shows delayed gastric emptying in patients with amyotrophic lateral sclerosis. Digestion 60 (6),567-571.
Further reading
Motor Neurone Disease Association. www.mndassociation.org
National Institute for Health and Care Excellence (NICE), (2016). Motor Neurone Disease: Assessment and Management. Available at: https://www.nice.org.uk/guidance/ng42/resources/ motor-neurone-disease-assessment-and-management-1837449470149.
Ng L, Khan F. Multidisciplinary care for adults with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No.: CD007425. DOI:10.1002/14651858.CD007425.pub2.
Sheffield Institute for Translational Neuroscience. www.sitran.org
This case study discusses the management of a patient with Motor Neurone Disease, who developed reflux and regurgitation on commencing gastrostomy bolus feeding. Despite the use of various enteral and oral feeds, his symptoms persisted, and he lost approximately 8% of his body weight in 9 months. After commencing Peptamen® AF, a 100% whey peptide formula with 50% of fat fr...






