
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease characterised by patchy, transmural inflammation and ulceration affecting anywhere in the gastrointestinal tract with ileocolonic disease being the most frequent presentation.1 While the exact cause of CD is not known, it is thought to result from a complex interplay between intestinal bacteria and environmental triggers (including diet) in genetically susceptible individuals.2
CD follows an unpredictable relapsing and remitting time course with acute exacerbations interspersed with periods of remission or less active disease.3 Typical symptoms include abdominal pain, diarrhoea and weight loss. Fatigue, anaemia and malnutrition are also common. Inflammation may also occur in other areas of the body and can affect the joints, skin, and eyes. Primary treatment aims are to induce and maintain remission. Management options include drug therapy, attention to nutrition, smoking cessation and in severe or chronic active disease, surgery.4 Corticosteroids, amino salicylates, antibiotics, immunosuppressives and biological agents are the mainstay of medical management. There is evidence to suggest that enteral nutrition (EN) can be used as a primary treatment or as an adjunctive treatment for induction and maintenance of remission.5 In conjunction with improving overall nutritional status, EN has been shown to induce remission and mucosal healing, improve mucosal permeability, down regulate pro-inflammatory cytokines and reduce serum inflammatory markers.6-9 Current guidance recommends that EN in adults be used as an adjunctive treatment to medical therapy to support nutritional support rather than an primary treatment except where patients decline other drug therapy.10,11,12
Patient’s Background
July - August 2012
A 28 year old male presented to his GP with a two week history of loose stools (bowels opening six to eight times a day), mouth ulcers and raised inflammatory markers (CRP 51). He had no other medical history and no family history of bowel pathology. He was a non smoker with minimal alcohol intake. He was referred for gastroenterology review. Weight: 90.4kg Height: 1.85m BMI: 26kg/m2
October 2012 - December 2012
A colonoscopy showed multiple aphthous ulcers on the left colon and histology consistent with the diagnosis of CD. Loose stools, mouth ulcers and raised inflammatory markers (CRP 40-50) continued with lethargy, poor appetite and loss of weight.
Pentasa 2g twice daily was commenced.
An MRI small bowel showed multiple distal small bowel strictures. A perianal abcess developed which was drained as an in-patient admission and a course of metronidazole 400mg three times daily was prescribed.
January 2013
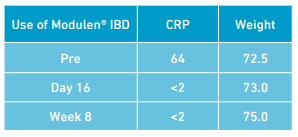
Symptoms continued with further loss of weight, poor appetite and raised inflammatory markers (CRP 64). Referral was made to the dietitian to consider adjunctive enteral nutrition to help mucosal healing. Mercaptopurine 50mg once daily was prescribed, however not commenced due to an issue with the prescription, in addition to Pentasa
2g twice daily. Weight: 72.5kg BMI: 21kg/m2
On initial dietetic consultation loss of weight was attributed to a combination of dietary changes (healthy eating) on diagnosis of CD and poor appetite. A 24 hour dietary recall revealed missed meals and oral intake estimated at approximately 50% of estimated nutritional requirements. The use of enteral nutrition as a treatment in CD was discussed and decided upon. A polymeric formula (Modulen® IBD) was commenced as an exclusive source of enteral nutrition for 8 weeks. The Modulen® IBD was established using a step up regimen to meet full estimated nutritional requirements over 4 days. The full volume regimen was tolerated with no adverse side effects. On day 8 of Modulen® IBD, lethargy had decreased, abdominal pain improved and stool frequency reduced (bowels open 2-3 times per day, consistency remained loose). He reported he was missing food! Mercaptopurine 50mg once daily commenced. On day 16 of Modulen® IBD; stool frequency reduced (bowels open 2 times per day), energy levels increased, no abdominal pain, wind and bloating resolved, and inflammatory markers decreased (CRP <2). He reported “feeling back to normal and back to normal routine”. Weight: 73 kg BMI: 21kg/m2
March 2013
At the end of the 8 week period of Modulen® IBD as an exclusive source of enteral nutrition, no symptoms were reported with an increase in weight. CRP <2. The Mercaptopurine was increased to 75mg once daily and Pentasa decreased to 1g twice daily. Weight: 75kg BMI: 22kg/m2
Food reintroduction began using exclusion diet3 with Modulen® IBD titrated against estimated oral intake to ensure nutritional requirements were met. No problems were encountered with food reintroduction until dairy products which appeared to cause abdominal discomfort. Dairy products were excluded and rechallenged at a later stage.
July 2013
No symptoms were reported with bowels opening up to a maximum of 3 times per day with a formed stool. 24 hour dietary recall showed oral intake was sufficient to meet nutritional requirements and dairy produce had been reintroduced with no reported problems. Modulen® IBD continued (1 drink) at breakfast time due to patient choice.
Weight 76.7kg BMI: 23kg/m2
No further dietetic follow up is planned. The patient has dietetic contact details for any queries and has a six month review planned with the gastroenterologist.
Nutritional Problems and Dietetic Intervention:
Symptoms:
Weight loss ( 20% in 6 months )
Insufficient intake for estimated requirements
Raised inflammatory markers
Daily nutritional requirements were estimated at 2,314 kcal, 78-91g protein and 2,555ml.13
Aims:
To meet nutritional requirements
To prevent further weight loss
To help alleviate clinical symptoms in combination with medical therapy
To reduce inflammation and induce remission
The volume of Modulen® IBD was determined using estimated nutritional requirements. Modulen® IBD was mixed to a 1.0kcal/ml dilution and introduced over a 4 day period to meet requirements. Total volume per day: 10 x 250ml drinks which provided 2,500kcal and 90g protein.
Rationale:
First line medication (Pentasa) did not adequately control inflammation and second line medication (mercaptopurine) can take 6-12 weeks to take affect.14 With significant loss of weight, poor appetite and continuing clinical symptoms, the multidisciplinary team felt that the patient would benefit from EN in adjunction to medical treatment for nutritional support and, to act as a ‘bridge’ to control symptoms before mercaptopurine took effect. The gastroenterologist was keen to promote mucosal healing via EN. Current guidelines recommend EN as an adjunctive treatment to support nutrition.10,11,12 To improve compliance and enable the patient to make an informed decision education was provided on elemental, semi-elemental and polymeric formulas and likely duration of EN. The majority of studies comparing elemental, semi-elemental and polymeric formulas have found equal efficacy.5
The patient was highly motivated to try EN to prevent further weight loss and improve clinical symptoms. He had a personal interest in the nutritional management of CD. Samples of elemental and polymeric (Modulen® IBD) formula were provided. Modulen® IBD is a casein based formula which is naturally rich in transforming growth factor-beta (TGF-ß2), an anti-inflammatory cytokine. Modulen® IBD has been shown to reduce local and systematic inflammation,6,15 promote weight gain and is associated with a remission rate of between 60-80%.6 Indications for the use of Modulen® IBD include: small bowel and colonic disease, stricturing disease and acute flare up16 – the patient met these criteria.
After trying the products the patient chose Modulen® IBD as an exclusive source of nutrition due to taste preference. Polymeric formulas may be advantageous in terms of palatability (which may increase compliance) and lower osmolarity.17
Modulen® IBD has minimal side effects (nausea, green stools, tiredness, hunger, flatulence, bloating, headaches, dizziness)18 when compared to the numerous side effects (weight gain, acne, moon face, mood disturbance, insomnia, hyperglycaemia, hypertension) and longer term health consequences (obesity, diabetes, depression, osteoporosis) of corticosteroids.3
The Modulen® IBD was built up over 4 days to minimise any side effects and used as an exclusive source of nutrition for an 8 week period. This 8 week period correlated with joint gastroenterologist and dietetic review. The optimal regime period as an exclusive source of nutrition is between 6-10 weeks to allow for mucosal healing.15, 19, 20
Results achieved using Modulen® IBD as adjunctive therapy:
- Nutritional requirements met
- Inflammatory markers decreased
- Weight maintenance and gain
- Improvement of symptoms
- Good compliance with no side effects
Induced remission in conjunction with medical therapy
Conclusion:
Loss of weight and malnutrition are common in CD. Many patients with CD struggle to manage oral diet to meet nutritional requirements and require nutritional support. EN should be considered in adjunction to medical treatment to optimise treatment results.
In this case EN in the form of Modulen® IBD was well tolerated and achieved optimum results. Modulen® IBD should be considered as a sole source of nutrition or in supplement to oral intake in adjunction with medical treatment to nutritionally support this patient group. A comparison cannot be made as no other product was used.
References: 1. Sands, BE. (2004) From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 126 (6): 1518-1532. 2. Sartor, RB. (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol hepatol. 3 (7): 390-407. 3. Lee J, Allen R, Ashley S , Becker S, Cummins P, Ghadamosi, Gooding AO, Huston J, Le Couteur J, O’Sullivan D, Wilson S, Lomer MCE. (2011) Evidence-based practice guidelines for the dietetic management of Crohn’s disease in adults. British Dietetic Association Birmingham. 4. National Institute for Health and Clinical Excellence. (2012) Crohn’s disease: Management in adults, children and young people. Clinical Guideline 152. London: National Institute for Health and Clinical Excellence. 5. Zachos M, Tondeur M, Griffiths AM. (2007).Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database of Systematic reviews. 6. Fell J, Paintin M, Arnaud-Battandier F, et al. (2000) Mucosal healing and a fall in mucosal pro-inflammatory Cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 14(3): 281-289. 7. Beattie RM, Schiffrin EJ, Donnet-Hughes A, et al. (1994) Polymeric nutrition as the primary therapy in children with small bowel Crohn’s disease. Aliment Pharmacol Ther. 8 (6): 609-615. 8. Bannerjee K, Camacho-Hubner C, Babinska K, et al. (2004) Anti-inflammatory and growth stimulating effects precede nutritional restitution during enteral feeding in Crohn’s disease. J Pediatr Gastroenterol Nutr. 38(3): 270-275. 9. Jones S, Shannon H, Srivastava E, Haboubi N. (2004) A novel approach to a patient with Crohn’s disease and a high output stoma: a missed opportunity? Scand J Gastroenterol. 39 (4): 398-400 [PubMed}. 10. Dignass A, Van Assche G, Lindsay JO, Lemann M, Soderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollon F, Hommes DW, Michetti P, O’Morain C, Oresland T, Windsor A, Stange EF, Travis SPL for the European Crohn’s and Colitis Organisation (ECCO). (2010) The second Euorpean evidence-based consensus on the diagnosis and management of Crohn’s disease: Current Management. Journal of Crohn’s and Colitis 4: 28-62. 11. Mowat C, Cole A, Winsdor A, Ahmed T, Arnott I, Driscoli R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho G, Satsangi J, Bloom S on behalf of the IBD Section of the British Society of Gastroenterology (2011). Guidelines for the management of inflammatory bowel disease in adults. Gut 60: 571-607. 12. Loch H, Dejongb C, Hammarqvistc F, et al. (2006) ESPEN (European Society for Parenteral and Enteral Nutrition) Guidelines on Enteral Nutrition: Gastroenterology. Clinical Nutrition. 25 (2): 260-274. 13. Todorvic VE, Micklewright A. (2011) A pocket guide to clinical nutrition on behalf of the Parenteral and Enteral Nutrition Group of the British Dietetic Association. 14. Crohn’s and Colitis Association availableat:http://www.crohnsandcolitis.org.uk/resources/CrohnsAndColitisUK/Documents/Publications/Booklets/Drugs%20 Used%20in%20IBD.pdf. 15. Borrelli O, Cordischi L, Cirulli M, Paganelle M, Labalestra V, Uccini S, Russo PM, Cucchiara S. (2006) Polymeric diet alone versus corticosteroids in the treatment of active Crohn’s disease: a randomised controlled open-label trial. Clin Gastroenterol hepatol. 4(6): 744-53. 16. Zaremba K, Falconer J. (2013) A guide for healthcare professionals: using Modulen IBD as a liquid diet. Nestle Health Science. 17. Thomas B, Bishop J. (2007) Manual of dietetic practice. 4th edition. Oxford:Blackwell Publishing; 2007. 18. Lee J, McGeeney L. (2008) Liquid diets and adult Crohn’s disease: what is current practice? Complete Nutriton. 8(2): 15-17. 19. Bascietto C, Borrelli O, Nardo G, Ambrosini A, Cirulli M, Bosco S, Cucchiara S. (2004) J Pediatr Gastroenterol Nutr. 39 Suppl 1:S106-7. 20. Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P and Russell RK. (2009) The use of exclusive enteral nutrition for induction of remission in children with crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment Pharmacol Ther. 30: 501-507.
Crohn’s disease (CD) is a chronic inflammatory bowel disease characterised by patchy, transmural inflammation and ulceration affecting anywhere in the gastrointestinal tract with ileocolonic disease being the most frequent presentation.1 While the exact cause of CD is not known, it is thought to result from a complex interplay between intestinal bacteria and environmenta...


