
1. Mucosal Healing
And a fall in mucosal pro-inflammatory cytonkine mRNA induced by a specific oral polymeric diet in paediatric Crohn's Disease
Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, Donnet-Hughes A, MacDonald TT, Walker-Smith JA. (2000) Aliment Pharmacol Ther. 14(3):281-9.
Background
Although enteral nutrition is a recognised form of treatment for intestinal Crohn’s Disease (CD), there are persistent problems with palatability and data are limited as to its mode of action.
Objective
In this prospective study, the effects of Modulen® IBD on the mucosal inflammatory process were evaluated in 29 children with active intestinal CD. All children were treated with Modulen® IBD as the sole source of nutrition for 8 weeks. Outcomes include clinical remission (defined as PCDAI <10), weight gain, endoscopic and histologic response, and markers of inflammation (CRP, TNF- ). Cytokine mRNA was measured in mucosal biopsies before and after treatment by quantitative reverse transcriptase polymerase chain reaction.
Results
- 96% of patients managed to take the polymeric formula orally
- The median PCDAI, initially at 30 declined to a median of 25 at 8 weeks (p < 0.00001).
- Children gained weight in response to treatment (mean weight gain at 8 weeks 3.2 kg, p < 0.001).
- After 8 weeks, 79% of children were in complete clinical remission (PCDAI <10).
- Macroscopic and histological healing in the terminal ileum and colon were associated with a significant decline in ileal and colonic IL-1ß mRNA (p = 0.006).
- IFN- mRNA before treatment was elevated in the ileum and colon when compared with controls (p = 0.004, p = 0.001). Levels fell in the ileum in response to treatment, with a ratio of pre- to post-treatment IFN- mRNA of 0.15 (p < 0.001). On the other hand, ileal TGF-ß1 mRNA rose with treatment (ratio 10, p = 0.04). In the colon there was no significant change in either IFN- or TGF-ß1 mRNA in response to treatment.
- In the colon IL-8 mRNA fell with treatment (ratio 0.06, p < 0.05).
- Over the 10 months follow-up period 9/23 cases (39%) who had achieved a clinical remission (PCDAI <10) relapsed (defined as a rise in PCDAI above 10).
Conclusion
The clinical response to Modulen® IBD is associated with mucosal healing and a down- regulation of mucosal pro-inflammatory cytokine mRNA in both the terminal ileum and colon. In the ileum there is also an increase in TGF-ß1 mRNA.
2. Effect of Enteral Nutrition
On Antioxidant Enzyme Systems and Inflammation in Paediatric Crohn's Disease
Phylactos AC, Fasoula IN, Arnaud-Battandier F, Walker-Smith JA, Fell JM. (2001) Acta Paediatr. 90(8):883-8.
Background
Crohn’s Disease (CD) is characterised by chronic inflammation of the gastrointestinal mucosa, which can be successfully treated with enteral nutrition. The activity of the antioxidant metallo- enzymes is significantly lower in children with CD than healthy children..
Objective
The purpose of this prospective study was to understand the mechanism of action of EN by assessing whether EN restores the activities of the antioxidant metalloenzymes copper/zinc- superoxide dismutase (Cu/Zn-SOD) and selenium-glutathione peroxidase (Se-GPx) in children with active CD. Children with active CD (PCDAI>10) (n=14) were treated with Modulen® IBD as the sole source of nutrition for 8 weeks. Measured outcomes include clinical remission (defined as PCDAI <10), antioxidant metalloenzymes, Cu/Zn-SOD and Se-GPx, and markers of inflammation (CRP and TNF- ).
Results
- In response to treatment, 13/14 patients went into complete remission. Overall, the PCDAI fell from an initial median of 26 to 0 (p < 0.001).
- Patients treated with Modulen® IBD did not have significantly altered Cu/Zn-SOD and Se-GPx enzyme activities.
- CRP fell from a median of 20 to 1.0 mg/L (p < 0.001), and serum TNF- from a mean of 12.8 pg/mL to 9.6 pg/mL (p < 0.05).
Conclusion
The results imply that the anti-inflammatory action of enteral nutrition in CD is caused by a mechanism other than restitution of these antioxidant enzymes.
3. Nutritional therapy Alone
With a Polymeric Diet (Modulen) is More Effective Than Corticosteroids in Inducing Healing of Intestinal Mucosal Lesions in Active Crohn's Disease
Bascietto C, Borrelli O, Di Nardo G, Ambrosini A, Cirulli M, Bosco S, Cucchiara S. (2004) J Pediatr Gastroenterol Nutr. 39 Suppl 1:S106-7.
Background
Therapy of active Crohn’s Disease (CD) should be aimed at obtaining clinical remission, improving quality of life and healing gut inflammatory lesions. The latter is a critical factor to provide long term remission. Traditional treatment for active paediatric CD includes corticosteroids or nutritional therapy alone; however, very few studies have compared the two treatment regimens taking into account inflammatory lesions of the gut as documented by endoscopy and histology.
Materials and Methods
This prospective study compared the efficacy of an oral polymeric diet (Modulen® IBD), rich in Transforming Growth Factor ß2, versus oral corticosteroids in inducing clinical and inflammatory remission in children with a newly diagnosed or relapsed CD. Thirty-two children with CD were randomised to receive either a Polymeric diet (n=17) (Modulen® IBD) exclusively for 10 weeks or oral corticosteroids (methylprednisolone 1.6 mg/kg/day and azatioprine) (n=15) for 4 weeks. Outcomes measured include clinical remission rate (PCDAI <10) and inflammatory remission which was defined as a decrease in both endoscopic and histological scores by more than 50% when compared to baseline.
Results
- The PCDAI improved both in the nutrition group and steroid group (p < 0.01 for both groups compared to baseline).
- However, endoscopic and histologic score significantly decreased only in the nutrition group (p < 0.01), whereas no significant change was observed in the steroid group
- Inflammatory remission was obtained in 82.35% of the patients on enteral nutrition and only in 40% of the patients on steroids (p < 0.01) (Fig).
Conclusion
In children with active CD, exclusive treatment with an oral polymeric diet is as efficacious as an oral corticosteroid course in inducing clinical remission. However, only nutrition therapy was shown to promote healing of gut inflammatory lesions as evidenced by endoscopy and histology scores. This is also in line with NICE Crohn’s disease management in adults, children and young people (Oct 2012) where mucosal healing may be better following treatment with enteral nutrition. Exclusive nutrition therapy is therefore a recommended treatment of choice in children with active CD. Modulen® IBD therapy may aid in mucosal healing.
4. Colonic Crohn's Disease
In Children Does Non Respond Well to Treatment with Enteral Nutrition if the Ileum is not Involved
Afzal NA, Davies S, Paintin M, Arnaud-Battandier F, Walker-Smith JA, Murch S, Heuschkel R, Fell J. (2005) Dig Dis Sci. 50(8):1471-5.
Background
Data supporting a response to treatment with exclusive enteral nutrition in paediatric colonic Crohn’s Disease (CD) are scarce.
Materials and Methods
This prospective study evaluated the changes in clinical and biochemical responses of ileal, colonic, and ileocolonic CD in 65 children with acute intestinal CD (PCDAI >20). The children were distributed into 3 groups:
- Colonic (n=14): involvement of any colonic location between the cecum and rectum with no small bowel or upper gastrointestinal involvement.
- Ileal (n=12): limited to the ileum, with or without spillover into the cecum.
- Ileocolonic (n=39): ileal disease and involvement of any colonic location between the ascending colon and the rectum.
Results
- At enrolment, the ileal group had significantly less severe disease (p = 0.05) compared to the colonic and ileocolonic groups.
- However, the smallest decrease in PCDAI scores at completion of treatment with enteral nutrition was observed in the colonic disease group (p = 0.03). The colonic disease group also had the lowest remission rate (50% vs. 82.1% in the ileocolonic and 91.7% in the ileal group) (p = 0.021).
- Endoscopic and histologic colonic mucosal assessment showed a post-treatment improvement in the ileocolonic (p ≤ 0.01) but not in the colonic disease group (p = NS) (Table).
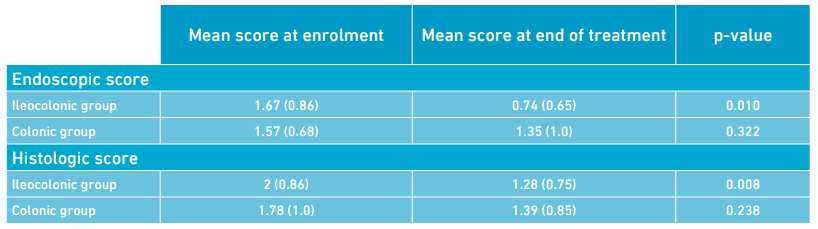
Table: Comparison of the colonic histologic and endoscopic scores in the ileocolonic and colonic groups shows significant improvement in the ileocolonic but not in the colonic group
Conclusion
Children with ilecolonic disease respond better to EN therapy than those with solely colonic or ileal involvement.
5. Energy Intakes of Children
With Crohn's Disease Treated with Enteral Nnutrition as Primary Therapy
Gavin J, Anderson CE, Bremner AR, Beattie RM. (2005) J Hum Nutr Diet. 18(5):337-42.
Background
Enteral nutrition (EN) is widely used and is effective in the treatment of children with Crohn’s Disease (CD) when given as an exclusive feed for 6-8 weeks. Current dietetic practice during EN is to recommend an energy intake based on the estimated average requirement (EAR) for energy according to age.
Materials and Methods
In this retrospective study, factors affecting energy intake and weight gain during EN in relation to disease site and nutritional status were examined. Forty children with CD were treated with Modulen® IBD alone for 8 weeks. Outcomes measured include weight gain, CRP (inflammatory marker), and energy intakes compared to EAR.
Results
- All patients gained weight during EN (median percentage weight gain is 11%).
- All patients showed a significant improvement in the CRP levels, suggesting a decrease in inflammatory response (p < 0.0001).
- Energy intake was higher than EAR in 82% of patients with a median of 117.5% of EAR.
- Weight gain correlated with Body Mass Index Standard Deviation Score (BMI SDS) (p = 0.001) at the start of treatment, but not energy intake or CRP. Those with lower BMI SDS at the start of treatment gained more weight during exclusive EN.
- No statistically significant differences in outcomes were seen in relation to disease site (small bowel, ileocolonic or colonic).
Conclusion
EN allows children with CD to gain weight in correlation with the BMI standard deviation score at the start of treatment. Energy intakes may be underestimated by EAR in children newly diagnosed with CD. Therefore, energy intakes of 100-149% EAR may be required.
6. Enteral Nutrition and Microflora
In Pediatric Crohn's Disease
Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L (2005) JPEN. 29 (4 Suppl):S173-5; discussion S175-8, S184-8.
Background
Exclusive enteral nutrition (EN) is an established primary therapy for paediatric Crohn’s Disease (CD). The mechanism of action of such treatment is still conjectural. Proposed mechanisms have included the elimination of dietary antigen uptake, overall nutrition repletion, provision of important micronutrients to the diseased intestine, correction of abnormal intestinal permeability, and immunological downregulation.
Materials and Methods
The purpose of this prospective study was to determine if EN-induced remission is associated with modification of fecal microflora in CD. Five healthy children and nine children with active CD were enrolled in the study. The children with active CD received Modulen® IBD only (n=9) for 8 weeks. At the end of the exclusive EN course, children returned to a free diet but continued to take 40% of daily caloric intake as polymeric formula. Eight of the nine patients also received the immunosuppressive agent 6-mercaptopurine. Outcomes measured include clinical remission (PCDAI <15) and fecal microflora.
Results
- In 8 of 9 children (88%), the exclusive EN alone induced disease remission (PCDAI <15) within 2 weeks of exclusive diet (Fig). All children were in disease remission after 4 weeks of exclusive EN.
- After the 8-week course of an exclusive liquid diet, patients on partial EN were followed up for a period ranging from 2 to 8 months. All patients maintained disease remission during the follow-up period.
- Profound changes in fecal microfora profiles were observed after treatment with exclusive EN compared to baseline.
- In contrast, control healthy children showed a host-specific and stable fecal microflora over time.

Conclusion
A possible mechanism of action of EN in inducing disease remission in CD may be its capacity to alter gut microflora.
7. Polymeric Diet Alone
Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: a Randomized Controlled Open-Label Trial
Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. (2006) Clin Gastroenterol Hepatol. 4(6):744-53.
Background
Nutrition therapy has been reported to have an almost equivalent efficacy as corticosteroids in achieving clinical remission in active Crohn’s Disease (CD). However, the effects of both treatments on intestinal mucosal inflammation are rarely reported.
Objective
The purpose of this prospective study was to compare the efficacy of exclusive nutrition therapy or corticosteroids on clinical variables and intestinal mucosal healing.
Thirty-seven children diagnosed with CD were randomised in an open-label trial to receive either Modulen® IBD alone (n=19) or oral corticosteroids (methylprednisolone) (n=18) for 10 weeks. Clinical remission (PCDAI <10) and mucosal healing (defined as a decrease in both endoscopic and histologic scores by 50% or more) outcomes were measured.
Results
- At week 10, the proportion of patients achieving clinical remission was comparable between the 2 groups (Modulen® IBD: 79%; corticosteroid group: 67%) (p = 0.4) (Fig).
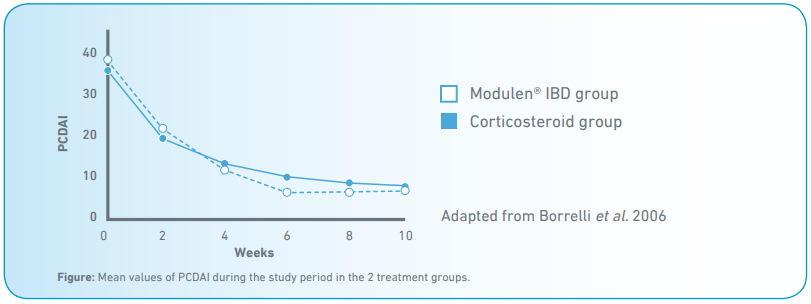
- On the contrary, the proportion of children showing mucosal healing was significantly higher in the Modulen® IBD (74%) than in the corticosteroid group (33%) (p < 0.05).
- At week 10, both endoscopic and histologic scores significantly decreased only in the Modulen® IBD group (p < 0.001).
Conclusion
In children with active and recently diagnosed CD, a short course of polymeric diet is more effective than corticosteroids in inducing healing of gut inflammatory lesions.
8. The Use of Exclusive
Enteral Nutrition for Induction of Remission in Children With Crohn’s Disease Demonstrates that Disease Phenotype Does Not Influence Clinical Remission
Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P and Russell RK (2009) Aliment Pharmacol Ther. 30: 501-507.
Materials and Methods
The purpose of this prospective study was to describe the centre’s experience of treating CD with a primary course of enteral nutrition and factors affecting treatment outcome. One hundred and fourteen children with CD were treated with either Modulen® IBD (n=105) for 8 weeks or with Elemental E028 extra (SHS international, Liverpool , UK) (n=5) for 8 weeks. Disease remission was assessed by several outcomes including patients’ well being, weight gain, stool frequency and inflammatory markers (CRP, ESR).
Results
- 52% of patients took feeds orally, 45% via nasogastric tube and 2% via PEG and 75% of patients completed at least 7 weeks of treatment.
- Clinical remission was achieved in 80% of patients.
- A significant decrease in both the median ESR and CRP at the start and end of treatment (38 vs. 13 and 22 vs. 6, respectively p < 0.001 for both) was observed in patients in clinical remission.
- Patients in clinical remission had significant improvements in weight and BMI Z scores at the end of the treatment (P< 0.001).
- The clinical remission rate in patients with isolated colonic disease was not different compared to other disease locations (77% vs. 79%, p = 0.88).
Conclusion
Enteral nutrition is well tolerated and results in clinical remission, normalisation of inflammatory markers, together with significant improvements in weight/BMI z-score in most patients. Clinical remission is not influenced by CD location suggesting enteral nutrition should be offered to all patients regardless of disease phenotype at diagnosis.
9. The Efficacy of Exclusive
Nutritional Therapy in Paediatric Crohn’s Disease, Comparing Fractionated Oral vs. Continuous Enteral Feeding
Rubio A, Pigneur B, Garnier-Lengliné H, Talbotec C, Schmitz J, Canioni D, Goulet O, Ruemmele FM (2011) Aliment Pharmacol Ther. 33:1332-1339
Background
Nutritional therapy has an established role as induction therapy in paediatric Crohn’s Disease (CD). However, patient compliance is an important challenge and may be greatly influenced by the administration route.
Materials and Methods
The objective of this study was to compare the efficacy of exclusive fractionated oral vs. continuous enteral feeding in inducing remission in children with CD. In this retrospective study, medical records of 106 children with active CD (PCDAI >10) were reviewed. All children were treated with Modulen® IBD as the sole source of nutrition for 8 weeks. The two comparative groups were oral intermittent or continuous enteral route by NG tubing. The primary outcome: Clinical remission defined as PCDAI <10.
Results
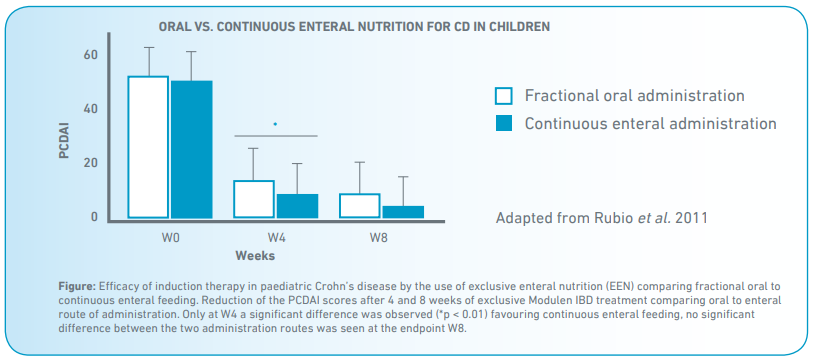
- At 8 weeks, 34/45 patients achieved remission in the oral group (75% on intention-to-treat analysis) and 52/61 (85%) in the enteral nutrition group (P = 0.157) (Fig).
- All patients showed a significant decrease in disease severity assessed by PCDAI (P < 0.0001) and significant improvements in anthropometric measures and inflammatory indices.
- Mucosal healing was observed and showed high correlation with remission.
Conclusion
Fractionated EN was shown to be as effective as oral nutritional therapy in inducing CD remission. All patients showed a significant decrease in disease severity and significant improvements in anthropometric measures and inflammatory indices. No differences were observed between Modulen IBD administered orally or by continuous enteral feeding, apart from weight gain which is greater in the enteral group. In a subgroup of patients (those who accepted follow-up endoscopy), mucosal healing was evident on follow-up endoscopies showing a clear correlation with remission. Compliance rates (87% oral group and 90% enteral group) were similar. Nevertheless, noncompliant patients had lower mucosal healing and remission rates.
Nutritional therapy based on the exclusivity principle is highly efficacious in inducing remission and mucosal healing in paediatric CD.
10. Influence of Exclusive
Enteral Nutrition Therapy on Bone Density and Geometry in Newly Diagnosed Paediatric Crohn’s Disease Patients.
Werkstetter KJ, Schatz SB, Alberer M, Filipiak-Pittroff B, Koletzko S (2011) JPGN. 52, (Suppl 2), E212.
Background
Paediatric Crohn’s Disease (CD) patients often present with deficits in muscle mass and bone quality at diagnosis and during follow-up. Exclusive enteral nutrition (EEN) induces remission and may have positive effects on muscle and bone.
Materials and Methods
The aim of this prospective study was to follow the development of muscle and bone in paediatric CD patients (n=10) initially treated with EEN (Modulen® IBD was given for 8 weeks, no steroids were applied) in the first year after diagnosis using peripheral quantitative computed tomography (pQCT).
Primary outcomes measured include disease activity (measured by PCDAI), bone mineral density: pQCT (XCT 2000, Stratec Inc.) at the non-dominant forearm (distal at 4%-site, proximal at 65%-site) and bone and muscle parameters: Trabecular (TrbD) & Cortical Density (CrtD), Total Cross Sectional Area (TotalCSA), Muscle Cross Sectional Area (MuscleCSA).
Results
- Disease activity decreased after EEN therapy.
- Low TrbD z-scores increased until week 12 (+0.3; (0.0-1.0); p=0.006) (Fig. 1).
- High CrtD z-scores normalised until week 12 (-0.4 (-1.1-0.5); p=0.027), but increased again between week 24 to 52.
- Z-scores of height corrected TotalCSA increased until week 12 (+0.2 (-0.2-0.6);p=0.014).
- Low height corrected MuscleCSA z-scores improved (+1.0 (0.6-1.8); p=0.002) until week 12 but remained on a low level compared to reference (Fig. 2).
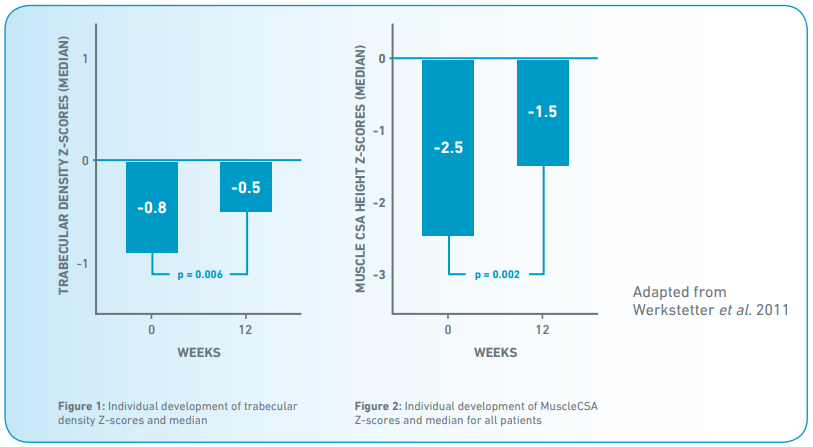
Conclusion
Low trabecular and high cortical bone density at diagnosis indicate disturbed bone remodelling. MuscleCSA is significantly impaired at diagnosis. Within 3 months after initiation of EEN therapy, bone metabolism and muscle mass significantly improve towards normalisation.
11. Two-Year Prospective Audit
Of Use of Liquid Diets as “Bridge” Therapy for Active Crohn’s Disease.
Demetriou T, Gawad C, Kok C, Harris AW, Gut (Supplement), March 2008;57:A1-A172
Background
Patients with active CD were prospectively recruited between April 2005 and August 2007.
Materials and Methods
Patients received Elemental E028 (SHS international , Liverpool UK) during the initial 8 months followed by Modulen® IBD for the remaining period. Exclusive enteral nutrition was followed for 4 weeks. Clinical response was assessed in clinic around day 28.
Results
- E028 was used in 13 patients, only 54% managed to complete 28 days treatment.
- Out of the Modulen® IBD group 69% managed to complete the 28 day treatment.
- Clinical remission was achieved in 6/7 in the E028 and in 9/9 in the Modulen® IBD group.
Conclusion
On an intention to treat (ITT) analysis a clinical response was achieved in 69% in the Modulen® IBD vs. 54% in the E028 group. Adherence can be achieved with well-motivated and supported patients. The use of liquid diets to avoid the use of steroids and to act as a “bridge” to allow the onset of action of thiopurine or methotrexate appears practical and effective.
12. Beneficial Effect
Of a Polymeric Feed, Rich In Tgf-Β, On Adult Patients With Active Crohn’s Disease: A Pilot Study.
Trianfillidis JK, Stamataki A, Gikas A, Sklavaina M, Mylonaki M, Georgopoulos F, Mastragelis A, Cheracakis P, Ann Gastroenterology 2006:19(1):66-71
Background
29 patients with active CD were treated with exclusive enteral nutrition using Modulen® IBD (50g x 5) for 4 weeks. Eight patients had enterocutaneous fistulae.
Materials and Methods
Activity of the disease was assessed at the beginning and after the 4 weeks by the use of Crohn’s Disease Activity Index (CDAI). Anthropometric, biochemical and clinical variables were also recorded. Medical treatment was left unchanged for the enteral nutrition treatment duration.
Results
- A clinical improvement was seen in 69% as per CDAI score.
- In addition, 4 out of 8 patients saw a 50% reduction in secretion of the fistulae after 4 weeks.
- One patient saw a complete closure of the fistula.
- Overall the feed was well tolerated with only one patient experiencing nausea and one experiencing diarrhoea as the recorded side effects.
- Reduction was seen in CRP and ESR levels.
Conclusion
This study shows that a polymeric diet rich in TGF-β could play a significant part in improving nutritional outcomes for adult patients with mild to moderately active Crohn’s disease.
This following shows a non-extensive list of the key clinical evidence behind Modulen® IBD. You will find a chronological summary of all the data collected and published over recent years that demonstrate the safety and efficacy of this product in Crohn’s Disease. Modulen® IBD is the only enteral product with extensive clinical evidence in Crohn’s Disease. All these studies have great...
Download content in PDF


