Introduction/overview
Chronic pancreatitis is a progressive and irreversible inflammatory disease of the pancreas. This results in exocrine and endocrine dysfunction which in time leads to maldigestion and malabsorption reducing the body’s ability to utilise essential macro and micronutrients. Consequently protein energy malnutrition is common. Research indicates that in more than 80% of cases this can be prevented with the use of pancreatic enzyme replacement therapy (PERT) and food alone. The addition of oral nutritional supplements will be required in 10-15% of patients, <5% will require tube feeding and <1% parenteral nutrition (PN).1,2
Patient’s background/medical history/physical/diagnosis
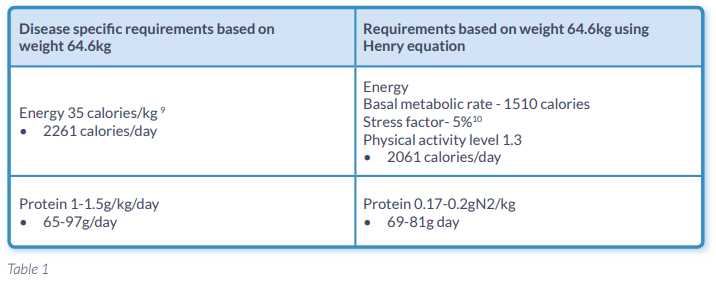
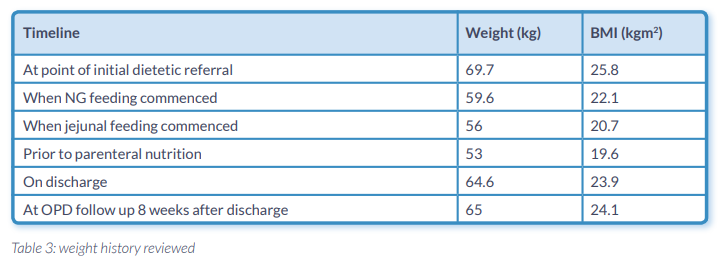
A 48 year old man with chronic pancreatitis was referred as an outpatient due to concerns of continued unintentional weight loss since recent major surgery. The patient had a trial dissection of a cystic lesion of the head of pancreas. This was abandoned and he had a double bypass with Roux-en-Y. There was no definitive evidence to suggest pancreatic cancer. When the patient was referred post operatively he had a total body weight loss of 12% since his surgery (Table 1.). Nutrition support in conjunction with pancreatic enzyme replacement therapy (PERT) was introduced.
The patient was very symptomatic with intractable abdominal pain, anorexia and steatorrhoea despite PERT, oral nutrition support and opiate analgesia. It became evident that enteral tube feeding was required to prevent further nutritional decline. NG feeding was trialled initially using a 1.0 kcal/ml peptide based formula (Nutrison Peptisorb, Nutricia UK). The patient’s stool frequency reduced with this intervention but the steatorrhoea continued despite titration of pancreatic enzymes (PE) and the addition of a proton pump inhibitor.3 The patient reported that despite an overall reduction in stool volume, his bowels continued to move both overnight and in the morning on completion of the enteral feed. The stool remained pale in colour and loose in consistency. He found urgency to open his bowels an extremely debilitating feature, and greatly impacting his ability to sleep overnight.
A 1.5kcal/ml peptide based formula (Vital 1.5kcal, Abbott UK) was also trialled but unfortunately had the same outcome as described above. The various strategies to try and overcome malabsorption are summarised in diagram 1.

Intractable abdominal pain refractory to analgesia led to an admission to hospital and it was during this admission the decision was made to progress to distal jejunal feeding. Jejunal feeding can achieve pancreatic rest as it does not stimulate pancreatic enzyme secretion and therefore minimises clinical symptoms.4,5
A jejunostomy feeding tube was placed surgically as this form of nutrition support was envisaged to be required long term. Feeding was commenced with a 1.0kcal/ml peptide based formula again alongside PERT. The patient reported his abdominal pain improved to some degree as it became tolerable with opiate analgesia, his bowel frequency reduced as did stool volume however the steatorrhoea persisted. The patient was discharged home as his weight stabilised.
Due to an acute exacerbation of abdominal pain and further weight loss the patient was admitted to the medical admissions unit. A new occurrence was pain at the site of the feeding jejunostomy. An imaging demonstrated stricturing proximal to the feeding tube and the patient was placed nil by jejunostomy.
The Nutrition Team made the decision to commence parenteral nutrition (PN) short term to prevent further decline. PN should only be considered when EN is not possible as although it provides full pancreatic rest it is associated with an increased risk of infection and metabolic disturbances.6
A summary of medical and nutritional problems identified on admission
- Faecal elastase 1 <50 ugE1/g.
- 24% body weight loss over 25 months with low BMI.
- Poor glycaemic control (on oral hypoglycaemic agents). It seemed compliance was an issue and no regular BM testing was being done at home.
- Negligible dietary intake due to prandial and postprandial symptoms.
- Recurrence of intractable abdominal pain despite opiate analgesia.
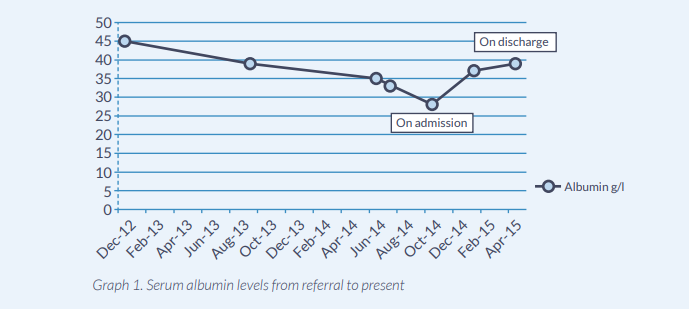
- Declining serum albumin (see Graph 1.), haemoglobin and ferritin.
Parenteral nutrition was commenced via a peripherally inserted central catheter (PICC) line and increased gradually over the following 5 days to prevent re-feeding syndrome and safely meet full nutritional requirements. A barium follow through was carried out during admission and no hold up was demonstrated at the site of the jejunal feeding tube. Due to the management of abdominal pain and the reassuring result from the barium follow through, small amounts of low fibre diet were reintroduced with PE. This is recommended as fibre may absorb enzmyes and delay absorption of nutrients.7 This was well tolerated but in small quantities only meeting up to 30% of nutritional requirements. The patient required insulin to maintain good glycaemic control as within 5 days of commencing PN blood glucose was up to ~19mmols. The target weight was achieved by week 8 and biochemical markers improved so the decision was made to trial jejunal feeding again as the only other alternative to maintain nutrition support at that stage would have been home parenteral nutrition.
As steatorrhoea had been persistent with the other formulas an alternative was indicated. Research suggested medium chain triglycerides (MCT) could be beneficial as they are absorbed directly across the small bowel to the portal vein.7,8 Peptamen HN® was the formula of choice as it is a peptide based formula with a high MCT:LCT ratio. The 1.33kcal/ml feed was chosen as it provides a higher calorie and protein content per ml optimal for cyclical feeding. This administration of feeding was the patient’s preference and clinical experience has proven its efficacy.1
Table 1. illustrates the patients nutritional requirements calculated using both standard and disease specific equations.
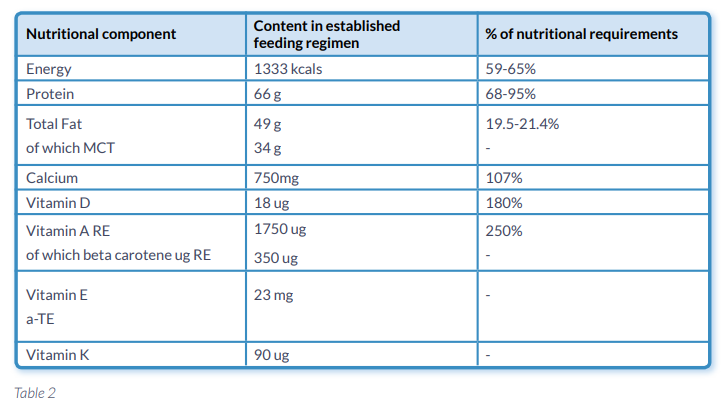
On day 1 Peptamen HN® was introduced at 10mls/hr and increased at 10ml/hr increments over the following 5 days. PN was reduced and discontinued by day 5 as the patient was meeting his full nutritional requirements. From day 2 the patient was passing formed stool with no evidence of malabsorption. On day 10 the patient was discharged as he was maintaining his weight and meeting his full nutritional requirements from 1000mls of Peptamen HN® overnight (Table 2.) with small frequent meals throughout the day. At 12 weeks following discharge the patient’s weight has increased marginally and his albumin has remained stable.
Outcomes/results achieved with Nestlé Health Science Product
The patient now passes formed stool with no evidence of malabsorption and urgency is no longer a feature. Abdominal pain is managed with opiate analgesia and has not resulted in further inpatient admissions to date (all of which were recurrent issues whilst using other enteral formulas). The overall outcome is that home parenteral nutrition has not been required, significantly minimising the risk and cost associated with nutrition support.
Although the patient still faces considerable challenges on a day to day basis, the newly established feeding plan has provided an improvement in his quality of life due to:
- An improved sleep pattern and less disruption to daily routine (as there has been no recurrence of the steatorrhoea)
- Less concern for the patient and his family due to sustained nutritional status
- Improved energy levels and appetite
From a medical and nutrition perspective the newly established feeding plan has:
- Improved pain management
- Improved glycaemic control (with the addition of insulin)
- Maintained nutritional status (Table 3.Graph 1.)
Discussion/hypothesis for success with Nestlé Health Science Product
We have demonstrated that the new artificial nutrition support feeding plan with a peptide and high ratio MCT:LCT formula has achieved maintenance of nutritional status for >12 weeks (Graph 1. Table 3.) in comparison to other peptide based formulas. This has been achieved by minimising fat malabsorption allowing for effective utilisation of macro and micronutrients. The patient also required insulin therapy to improve his glycaemic control. This required further titration on discharge demonstrating effective absorption on enteral nutrition. We can hypothesise that this was as a result of the lipid profile as the other formulas do not contain as high a MCT:LCT ratio as Peptamen HN®.
The patient continues with Peptamen HN, but requires considerably less PERT. The patient was previously requiring 4 hourly PERT 2 x 40,000u and now he takes only 1 x 40,000u at beginning of feed and one at end.
Peptamen HN® is nutritionally complete so I would be confident that this is an appropriate long term solution for providing nutrition support should artificial nutrition be required as the sole source of nutrition in the future. Research has suggested that long term jejunal feeding is thought to be safe, well tolerated and effective in those unresponsive to other forms of standard nutrition support.2
References:
- Meier R, Ockenga J, Pertiewickz M, et al. ESPEN Guidelines on Enteral Nutrition: Pancreas. Clinical Nutrition 2002; 21(2): 275-284.
- Duggan S, O’Sulivan M, Feehan S, Ridgway P, Conlon K. Nutrition treatment of deficiency and malnutrition in chronic pancreatitis: a review. Nutrition in Clinical Practice 2010; 25(4): 362-370.
- Heijerman HG, Lamers CB, Bakker W. Omeprazole enhances the efficacy of pancreatin (pancrease) in cystic fibrosis. Ann Intern Med 1991;114:200-1.
- Vu, et al. ‘Does Jejunal Feeding Activate Exocrine Pancreatic Secretion?’. European Journal of Clinical Investigation 29.12. 1999: 1053-1059.
- Stanga Z, Giger U, Marx A, DeLegge MH. Effect of jejunal long-term feeding in chronic pancreatitis. JPEN Journal Parenteral Enteral Nutrition. 2005; 29(1): 12-20.
- Lordan JT, Phillips M, Chun JY, Worthington TR, Menezes NN, Lightwood R, Hussain F, Tibbs C, Karanjia ND. A safe, effective, and cheap method of achieving pancreatic rest in patients with chronic pancreatitis with refractory symptoms and malnutrition. Pancreas 2009; 38(6): 689-692.
- Rasmussen, Henrik Højgaard. ‘Nutrition In Chronic Pancreatitis’. World Journal Gastroenterology 2013; 19.42: 7267.
- Caliari S, Benini L, Sembenini C, Gregori B, Carnielli V, Vantini I. Medium-chain triglyceride absorption in patients with pancreatic insufficiency. Scand J Gastroenterol 1996; 31: 90–4.
- Giger U, Stanga Z, DeLegge MH. Management of chronic pancreatitis. Nutrition Clinical Practice 2004; 19(1):37-49.
- Hebuterne X, Hastier P, Peroux JL et al. Resting energy expenditure in patients with alcoholic chronic pancreatitis. Digestive Disease Science 1996; 41: 533-539.
Introduction/overview Chronic pancreatitis is a progressive and irreversible inflammatory disease of the pancreas. This results in exocrine and endocrine dysfunction which in time leads to maldigestion and malabsorption reducing the body’s ability to utilise essential macro and micronutrients. Consequently protein energy malnutrition is common. Research indicates that in more than 80...