A practical approach to managing a CMA infant with Althera™
Cows’ Milk Allergy (CMA) can occur in 1.8% - 7.5% of infants in the first year of life.1 The allergy is defined as an adverse reaction to the proteins in milk with most children growing out of their allergy by the time they reach one year. The treatment consists of the exclusion of cows’ milk from the infant’s diet.
There are now suitable resources to help with the management of CMA in primary care, such as the MAP (Milk Allergy in Primary Care) guidelines which are an extension to the NICE guidelines “Food allergy in children and young people” (2011) and the BSACI guidelines, which can be accessed online.1,2
Patient’s medical history
Patient X was referred to the dietitians at the age of 11 weeks with a history of constipation and unsettledness, as well as reflux, but no vomiting. The patient had initially presented with these symptoms at approximately 2 weeks old and was seen as an outpatient alongside the paediatrician.
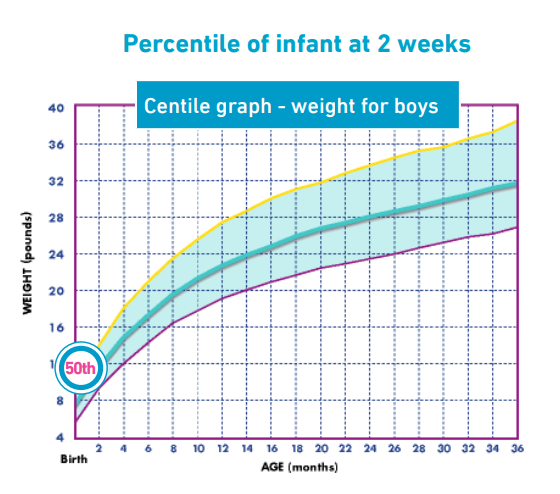
Patient X was currently on a standard formula managing 177mls/kg/day. Lactulose had been prescribed prior to appointment to help with the constipation. His weight was on the 50th centile and unfortunately height was unable to be recorded due to the patient’s distress.
An allergy focused history was taken and with an incidence of asthma in the family and non IgE symptoms suspected, an elimination diet was discussed with the exclusion of cows’ milk protein for 2 to 8 weeks. The theory being that if symptoms do not improve then cows’ milk protein allergy is unlikely.3
Nutritional Intervention
First line treatment for most cases of non IgE mediated reactions would be an extensively hydrolysed formula, they are cheaper and are generally well tolerated.3 In this case it was Althera™, an extensively hydrolysed formula (eHF) with the addition of lactose, that was prescribed. In the initial consultation I explained CMA to the parents and provided detailed information of the supplied formula, Althera™. I provided 3 sample tins of Althera™ and advised that the parents were to contact the dietitian directly to confirm if they felt that the patient showed improvements, and then a prescription could be arranged. I also advised the parents to reduce the formula volumes slightly to ensure that patient X received 150mls/kg/day as the reduction in volume may help with the reflux. No other changes to medications were made at this appointment.
Outcome
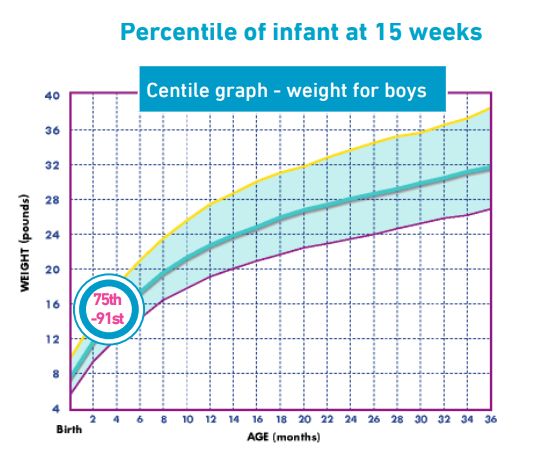
After 6 days the parents phoned to say that patient X was straining less to pass a stool and was noticeably more settled. I then reviewed patient X at 15 weeks (4 weeks later) as an outpatient alongside the paediatrician. His weight was now on the 75th-91st centile and his height on the 50th centile.
The parents reported that their child’s symptoms had shown great improvement, stools were being passed with ease, and the patient was much happier and taking Althera™ with good acceptability. Weight had increased greatly but volumes of Althera™ being taken were reported being 140mls/kg/day, which is just under the patient’s recommended fluid intake (Nutritional Requirements, Great Ormond Street Hospital for Children, NHS Foundation Trust, London). Due to good growth I recommended that the parents continue to use Althera™ and adjust the formula volumes accordingly, once they start weaning their child. For weaning preparation guidance I discussed dairy free weaning and suitable first foods.
Discussion
Guidelines now suggest to re-challenge the patient after a 2-4 week trial of a milk free diet2 but some suggest to continue with the exclusion diet if the trial has worked1 which was done in this instance.
Patient X will then be reviewed again by the dietitians at the age of one year, where re-introduction advice to cows’ milk will be given. In the meantime, health visitors in Grampian now have the knowledge to guide parents through dairy free weaning for mild to moderate cows’ milk allergy and if there were any concerns they could contact the dietitians.
Summary
Althera was well tolerated by the patient and symptoms of CMA were greatly improved. In addition the patient demonstrated positive weight gain and growth and continues on Althera in line with BSACI guidelines.1 Althera is a useful tool in the dietary management of mild to moderate CMA.
References:
- BSACI guideline for the diagnosis and management of cow’s milk allergy. D. Luyt, H. Ball, N. Makwana, M. R. Green, K. Bravin, S. M. Nasser and A. T. Clark. University Hospitals of Leicester NHS Trust, Leicester, UK.
- MAP Guidelines http://cowsmilkallergyguidelines.co.uk/
- LUDMAN, S et al. (2013) Managing cows’ milk allergy in children [Clinical Review]. British Medical Journal, 347:f5424 [Accessed 12/04/2014].
IMPORTANT NOTICE: Mothers should be encouraged to continue breastfeeding even when their infants have cows’ milk protein allergy. This usually requires qualified dietary counselling to completely exclude all sources of cows’ milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasising that unboiled water, unsterilised bottles or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.
A practical approach to managing a CMA infant with Althera™ Cows’ Milk Allergy (CMA) can occur in 1.8% - 7.5% of infants in the first year of life.1 The allergy is defined as an adverse reaction to the proteins in milk with most children growing out of their allergy by the time they reach one year. The treatment consists of the exclusion of cows’ milk from the infant’s diet. There...